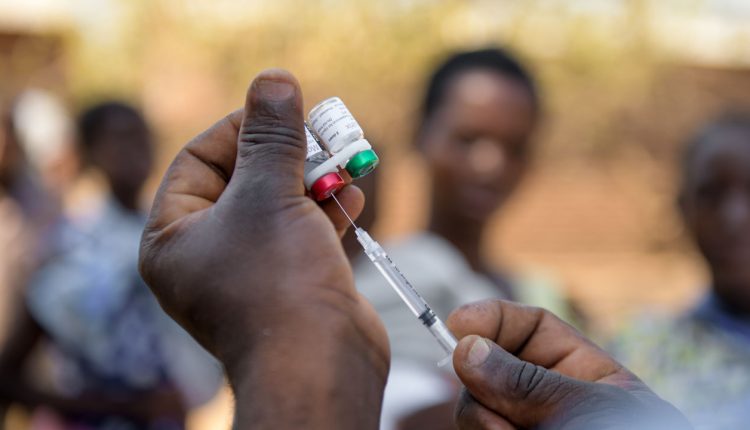
New malaria vaccine developed by scientists at the University of Oxford has been approved in Ghana and Nigeria this month, which is considered an important step in fighting the disease.
Ten more African countries are reviewing trial data for the vaccine, which has undergone clinical trials in the UK, Thailand, and several African countries, according to the World Health Organisation (WHO) and the University of Oxford.
Nigeria, which accounts for 31.3 percent of the total deaths of malaria worldwide has granted regulatory clearance for the rollout of the R21/Matrix-M vaccine on Tuesday, following Ghana.
Ghana and Nigeria have approved the vaccine for use on children of ages between five and 36 months, the highest age group at risk of death from the disease. The vaccine shot was found to reduce the risk of the mosquito bite disease by 77 percent.
“This marks a culmination of 30 years of malaria vaccine research at Oxford with the design and provision of a high efficacy vaccine that can be supplied at adequate scale to the countries who need it most,” said Professor Adrian Hill, chief investigator on the R21/ Matrix-M program.
“These vaccines can save lives and save hospitalisations, reduce the impact of the disease in the most vulnerable young children,” said Dyann Wirth, an infectious disease professor at Harvard T.H. Chan School of Public Health.
The malaria vaccine is being manufactured by the Indian Serum Institute, which suggested it has the capacity to supply 200 million doses per year, as the vaccine is cheap to produce and straightforward to transport.
“Malaria is a great drain on many national economies, especially as many poorer nations are among the most affected. As such, the disease maintains a vicious cycle of disease and poverty,” says the US Centers for Disease Control and Prevention.
In 2021, 619,000 people have reportedly died of malaria, the latest annual total available, and 96 percent of those deaths occurred in Africa, according to the WHO.