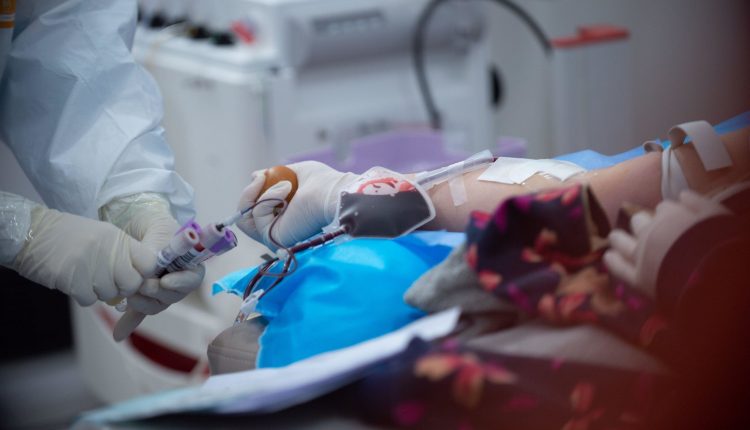
The U.S. Food and Drug Administration (FDA) approved on Thursday a clinical trial at Columbia University to determine whether the plasma collected from coronavirus survivors can effectively protect healthcare workers on the frontlines and ease severely-ill patients’ symptoms.
The study is financed by $2.5 million from Amazon.
Using convalescent plasma – a component of blood – as a treatment is a decades-old idea. It has already been used to treat other diseases such as influenza and measles. When a patient recovers from a disease, they tend to produce antibodies to combat the presence of the antigen that caused the disease in the first place, and those proteins will stay in the blood for a few months.
Researchers at Columbia hope that plasma from recovered coronavirus patients can be transfused into severely-ill patients, helping them recover more quickly. This plasma could also help healthcare workers on the frontlines develop some immunity.
It’s not an alternative to a vaccine, researchers say but they believe that the approach has potential.